Student Jeehyeon Lee theoretically uncovers the principle of inhibiting antibody aggregation
- Views 7804
- Writer 커뮤니케이션팀
- 보도일자 2021-02-08
Student Jeehyeon Lee (Department of Chemistry, ‘17) from Professor Sihyun Ham's lab in the Department of Chemistry at Sookmyung Women’s University revealed the principle of effectively inhibiting aggregation, which is considered a major obstacle in antibody therapy that is rapidly advancing in the development of new drugs. This research was supported by the Samsung Science & Technology Foundation and the National Research Foundation of Korea, and the results were published on December 3 in the sister magazine “Scientific Reports” of Nature, an internationally renowned academic journal. (Title of Paper: Local Environment Effects on Charged Mutations for Developing Aggregation-Resistant Monoclonal Antibodies.)

(Photo Description: Professor Sihyun Ham (left), Student Jeehyeon Lee (right))
Antibody therapy is widely used, such as in cancer, viral infection, and autoimmune disease, and is receiving more attention due to the recent pandemic COVID-19. However, there has been a problem in that the effect is deteriorated by easily agglomerating during high concentration injection and transportation processes. It has already been known that aggregation is less likely when a positively or negatively charged amino acid residue is introduced into an antibody (charged mutation), but development has been difficult due to lack of understanding in which amino acid residues should be introduced into which part of the antibody for each specific antibody.
Accordingly, Prof. Ham's research team calculated the solvation free energy of human immunodeficiency virus type 1 (HIV-1) antibodies and antibodies related to dementia, which showed experimentally improved solubility by applying a fluctuating thermodynamics analysis method. With this, the team analyzed how the introduced amino acid residues and their surrounding residues contribute to the solvation free energy of the antibody.
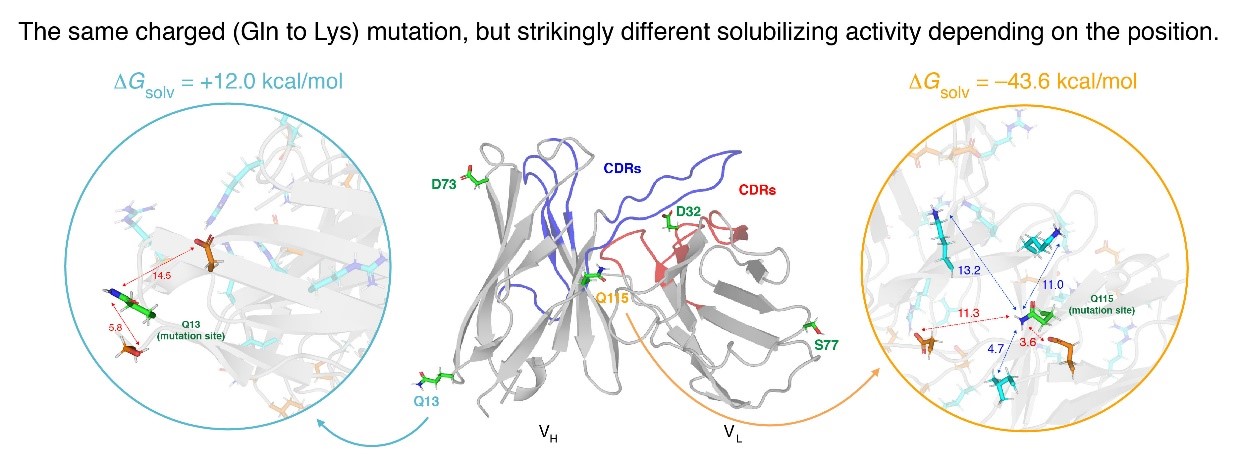
As a result, a local environment effect was found that antibody aggregation was dependent on the kind of residues located within about 15 Å from the introduced amino acid (charged mutation site) (See figure). This improved the understanding of the mechanism of antibody aggregation due to charged mutations in addition to the previous research by Professor Ham that the charge of amino acids introduced as mutations varies depending on the net charge of the protein and shows a large stable solvation free energy. Through this, it is expected to be used in various ways in the pharmaceutical area related to antibodies in the future.
Prof. Ham said, “Even though she is an undergraduate student, Jeehyeon Lee enthusiastically participated in the research, and as a result, I am proud that she was able to achieve such excellent results.” Student Jeehyeon Lee said, “I was able to participate in the study as an undergraduate student thanks to the lab’s excellent system. I think it was the biggest factor that made such good results possible, and I would like to apply it to various antibody therapy related researches in the future.”



