Prof. Wu-yeol Kim's team uncovers the world's first path to creating useful compounds from CO2
- Views 7004
- Writer 커뮤니케이션팀
- 보도일자 2020-12-01
In the midst of active e-chemical researches to turn carbon dioxide, which is the cause of climate change, into high value-added compounds based on new and renewable energy, researchers at Sookmyung Women’s University succeeded in identifying the key intermediate path of the ethylene production reaction also known as the “bread and butter of industries.” It is expected to be a breakthrough in responding to climate change and developing clean, next-generation carbon resource technology.
The research team of Professor Wu-yeol Kim of Sookmyung Women’s University’s Department of Chemical and Biological Engineering collaborated with the research team of Dr. Yun-jeong Hwang of the Clean Energy Research Center of the Korea Institute of Science and Technology (KIST, Director Seok-jin Yun) jointly revealed that they succeeded in observing the reaction intermediate adsorbed on the surface of copper-based catalyst during the process of synthesizing ethylene by reducing carbon dioxide, as well as analyzing its behavior in real time.
The results of this research have been published in the latest issue of the 「Energy & Environmental Science」, (IF: 30.289, the top 0.189% in the JCR field).
(Title of Paper) Time-resolved observation of C-C coupling intermediates on Cu electrodes for selective electrochemical CO2 reduction
- Lead authors: So-jeong Park, Ph.D. student in the Department of Chemical and Biological Engineering, Dr. Yeong-hye Kim of the Korea Institute of Science and Technology
- Corresponding author: Wu-yeol Kim, Professor of Department of Chemical and Biological Engineering, Senior Researcher Yun-jeong Hwang of Korea Institute of Science and Technology

(Photo from left) Ph.D. student So-jeong Park and Professor Wu-yeol Kim
According to Professor Wu-yeol Kim, it has been reported that copper-based catalysts can convert carbon dioxide to synthesize not only relatively simple carbon monoxide or formic acid, but also multi-carbon compounds such as ethylene, methane, and ethanol having two or more carbons. However, the major intermediates and pathways of the carbon-carbon bonding reaction have not been identified, so the development of control technology for selectively synthesizing high value-added compounds has been limited.
Accordingly, the research team applied infrared spectroscopy to observe the intermediate (OCCOH) in the process of becoming ethylene, in addition to carbon monoxide, during the carbon dioxide conversion reaction on the surface of the nanocopper particle catalyst and the intermediate (CHO) producing methane. As a result, it was found that carbon monoxide and ethylene intermediate (OCCOH) were produced at the same time, while methanol intermediate (CHO) was produced relatively slowly compared to the two intermediates. It confirmed that the control of the reaction path could further improve the selectivity of compound formation on the catalyst surface.
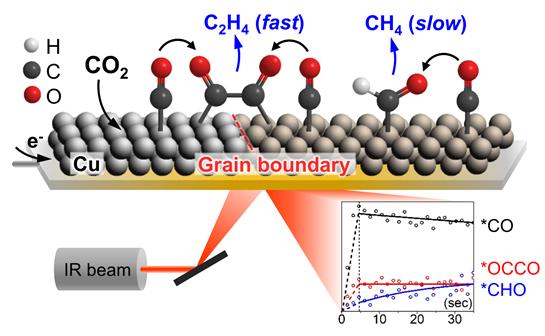
Along with this, they proposed a copper hydroxide nanowire as a new catalyst material that shows excellent performance in ethylene production by promoting carbon-carbon bonds. The research team was able to confirm that there are various sites on which carbon monoxide (CO) can be adsorbed on the surface of the catalyst derived from copper hydroxide, and carbon monoxide adsorbed at a specific site among them quickly forms a carbon-carbon bond intermediate. Further research is expected to contribute significantly to the identification of the active site of the carbon-carbon bonding reaction, which has been the subject of debates.
Professor Wu-yeol Kim said, “This research is expected to be an important step in the development of e-chemical technology that converts carbon dioxide, which is pointed out as the cause of climate change, into useful compounds,” and “It will be of high value as a climate change response technology if the follow-up researches at the stage of developing electrochemical reactor technology and plant process technology go smoothly after securing the source technology for catalyst materials.”



